| |


|
|
| |
|
STRUCTURE, FUNCTION AND ASSEMBLY OF MUCUS AND MUCINS IN THE GASTROINTESTINAL AND RESPIRATORY TRACTS.
RELATION THE HUMAN DISEASES: INFLAMMATORY BOWEL DISEASE, ULCERATIVE COLITIS, Crohn's DISEASE; IBS; CYSTIC FIBROSIS, BRONCHITIS, and COP
The group is studying the structure and function of mucins (mucus glycoproteins) and related components of mucus. This includes all aspects of mucins including their molecular structure and glycans. A special focus is on the gastrointestinal tract and the role of mucus and mucins in the protection of the intestine and as part of the intestinal innate immune system. This also includes mucins in relation to cystic fibrosis and colon cancer development. Some members of hte groups also have their focus on the respiratory tract and how this is kept essentially free of bacteria in the normal situation and how this is alterad at disease.
Mucins are extracellular large highly glycosylated molecules having mucin domains. Usually one also demands that there is more than 50% of the mass that is due to glycans. Mucin domains (with glycans) or PTS domains (protein core only) are found also in other extracellular proteins, but are dominating in molecules that are usually called mucins. Mucin domains are rich in the amino acids threonine, serine and proline, where the oligosaccharides are linked via N-acetylgalactosamine to the hydroxy amino acids. There are two types of mucins, secreted and membrane bound. Some of the secreted mucins are gel-forming due to their polymeric nature.
The major mucin of the gastrointestinal tract is called MUC2 and is produced by the intestinal goblet cells. The apoprotein of this mucin has two central mucin domains and cysteine-rich domains at both the N- and C-terminal ends. The primary translational product is about 600,000 kDa in mass and is very quickly dimerized in the endoplasmic reticulum of the cell. This dimer is formed in the C-terminus.The addition of O-glycans starts when the dimer enters the Golgi apparatus and when fully glycosylated the mass will increase to about 5 million Da. The MUC2 mucin is forming polymers in the late Golgi - TGN compartment. This involves two different processes. One is the formation of disulfide bonds between the N-termini and the other is the formation of isopeptide bonds between th eamino acids lysine and glutamine. Thus the MUC2 mucin is forming enormous net-like covalent polymers.
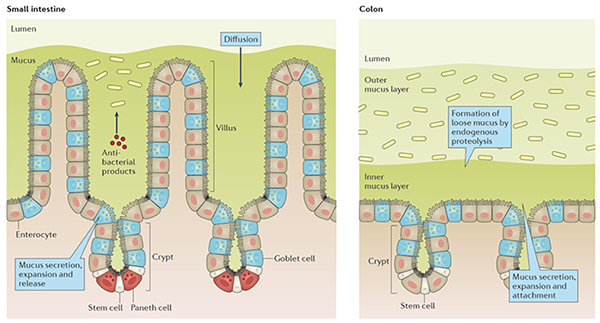 |
Fig. 1.
The The mucus layers of the small and large intestines. |
All human beings have more than two pounds of bacteria in their large intestine and the number of bacteria are similar to the total number of cells in the body. How we can live with all these bacteria without deleterious effects or diseases was not understood until we 2008 couold show that there is an inner mucus layers in colon that act as a barrier and separate us from the large number of bacteria. In the absence of the MUC2 mucin, the molecule that builds the mucus layers, the bacteria can reach the epithelial cells. These animals got an inflammation and later on colon cancer, a scenario that is similar to the human disease ulcerative colitis. During our studies of the mucus layers, we found a number of other molecules that are vital for the correct function and properties of the mucus. We study the goblet cells that form most of the mucus, the different mucus components that together with the MUC2 mucin form the mucus. We study the structure, modifications and properties all these components.
The transmembrane mucins are anchored in the apical cell membrane of epithelial cells of mucosal surfaces. These are forming a dense glycocalyx, especially on enterocytes of the small intestine. Most of these are long and extend around one micrometer from teh apical membrane into the lumen. The function of the transmembrane mucins is poorly understood, but in addition to protection they probably also act as sensors and signaling molecules. One of the groups are addressing their function in the intestinal tract with a special interest in their interactions with intracellular molecules.
The glycan parts of mucins are analyzed after release of the oligosaccharides as the mucins domains are too large to allow analysis of the attached glycans. Developments in methods and equipment has allowed the analysis of O-glycosylation from very small amounts of material, exemplified by the analysis of specific mucin glycosylation from millimeter-sized biopsies of human colon.
Members of the Mucin Biology Groups also study the respiratory system and its mucus. In the normal lung, thick mucus bundles are formed by the submucosal grands. These are sweeping the surface and in this way efficiently clean move debris and bacteria up to the larynx where it is swallowed. In the smaller airways lacing the submucosal glands, the mucus is forming mucus in the form of clouds. Upon infection or at Cystic Fibrosis, Chronic Bronchitis or COPD, an attached mucus layer is formed. This is trapping bacteria and can in this way protect the epithelial cells, just as in colon. In healthy inividuals, this mucus can be coughed up once the infection is under control.
>br>Expected results are novel ways to improve the protection of colon and by this treat ulcerative colitis and other inflammation related diseases. Futhermore, moelcular understanding of the respiratory mucus system will open for the developemnt of novel therapies for many lung disease. |
|
|
|
|
| |
|
|

